Parkinson’s Disease & Latest Research Initiatives
Posted by Phil Heler, MD on July 12, 2019Parkinson's Disease is the second most common neuro-degenerative condition in the UK and here I outline some exciting ne
Today’s post is about Parkinson’s disease. In May this year I wrote an article about ground-breaking gene silencing techniques and the huge potential to treat genetically inheritable diseases such as Huntington’s disease. What is very exciting is that it has just been approved for use by the NHS. The technique is being used to solve a previously untreatable rare condition called amyloidosis that affects about 150 people in the UK. Amyloidosis is the name for a group of rare, serious conditions caused by a build-up of an abnormal protein called amyloid in organs and tissues throughout the body.
There are many types of hereditary amyloidosis caused by various gene mutations. The build-up of amyloid proteins can make it difficult for organs and tissues to function properly and the condition can cause serious complications. This new treatment completely halts or even reverses the disease. The whole point about gene silencing however is its huge potential to solve other conditions. It was only very recently that Craig Mello and Andrew Fire, now at Stanford University School of Medicine in California, shared a 2006 Nobel Prize for discovering the process of silencing genes.
At the time this appeared to be almost pure science fiction, but it has now become a firm reality. What excites me is that this technique may have the possible capacity to tackle neuro-degenerative conditions such as Parkinson’s and Alzheimer’s. In the past 10 years, researchers have identified a handful of causal Parkinson’s genes (GBA, LRRK2, PRKN, SNCA and there may be many others), where genetic mutations greatly increase our risk. Parkinson’s is the second most common neuro-degenerative condition in the UK, and it is something I haven’t yet reviewed.
The Background behind Parkinson’s Disease
James Parkinson first gave a clinical description of the disease in 1817 in his study called ‘An Essay on the Shaking Palsy’. What I do find difficult to accept is that for many people the actual cause of Parkinson’s is unknown (or idiopathic) although current research appears to suggest that there is a combination of factors that may serve to interact. The best explanation that exists is that there appears to be a spectrum, with genetic factors at one end of the scale and environmental factors at the other, with the premise that any one individual case would fall somewhere in between. Current studies suggest that about 30% of the risk of developing Parkinson’s is attributable to genetic components but is also very probable that yet more Parkinson’s genes await discovery.
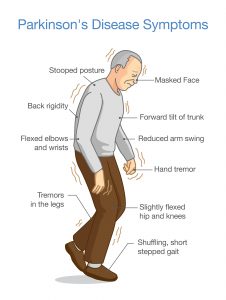
Above all else age itself appears to be the single greatest risk factor. Typically, people begin to exhibit symptoms when they are over 50 but 1 in 20 of us also exhibit symptoms when we are under 40 years old. After the age of 50 prevalence rises rapidly, so that by the end of our seventh decade an estimated 1 person in 200 have the disease, and by the end of our eighth decade the proportion is 1 in 40. Due to our ageing population profile, researchers project the number of people with Parkinson’s will double by 2040. There is no doubt that age-related processes lead to fundamental changes at cellular level.
Studies suggest our cells may be more susceptible to damage as they age and that over time the expression of our genes may change, which could set off a chain of cellular events that eventually leads to Parkinson’s disease (or PD). What we do know is that for PD to occur, neurons in the brain gradually break down or die. What is strange with PD is that there is a highly selective degeneration of different neuron types at various levels within brain tissue. It would appear to specifically target various points in the brain, or nuclei, that produce neurotransmitters. This leads to low levels of dopamine (causes loss of movement and tremor), glutamate (slowness of movement and cognitive function), norepinephrine (loss of motor movement) , serotonin (depression) and acetylcholine (loss of sense of smell).
How does Parkinson’s Affect us?
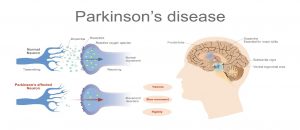
A typical healthy human brain has roughly 100 billion neurons. Clearly, as many of us know, neurons are specialised cells in our brain that transmit and receive signals using chemical and electrical pathways. Most cells in the human body live for a relatively short amount of time, but neurons can live up to 100 years. As a result of this longevity they undergo constant repair, maintenance and regeneration so we maintain our memory and learn. One of the most serious effects of PD is the very specific loss of neurons in an area of our midbrain called the putamen.
These cells produce a chemical messenger in our brain called dopamine. These dopamine producing neurons are part of the substantia nigra pars compacta nucleus and typically symptoms of PD become apparent when dopamine levels have decreased by about 70%. The neurons of the substantia nigra have a crucial role as they communicate with neurons of the basal ganglia by liberating dopamine. Such an interaction at the biochemical level is responsible for the fine tuning of movement. This is because the basal ganglia is associated with a variety of functions, including control of voluntary motor movements, as well as learning, eye movements, cognition, and emotion.
However, the neurodegenerative process of Parkinson’s Disease also selects other neuron groups distributed throughout our central nervous system including specific parts of in our brains such as our thalamus, brain stem, and spinal cord and our autonomic nervous system (or ANS). What is the ANS? The ANS consists of motor neurons that help control all smooth muscle in our bodies (smooth muscle lines the walls of all our organs), cardiac muscles, and glands that are responsible for secretions.
In addition, the ANS keeps tabs on all our organs, blood vessels and provides feedback to our brains. The thalamus meanwhile relays these sensory impulses from our ANS to the cerebral cortex where it is interpreted as touch, pain or temperature. PD specifically affects an area in the thalamus called the centromedian nucleus that uses glutamate as a neurotransmitter. It also affects three areas in the central cortex that also makes and uses glutamate for the same reason. For the sake of completion these are the parahippocampal gyrus (important for the storage of memories), insular cortex (helps with a sense of self-awareness) and the presupplementary motor area (helps control our actions).
There is also a significant loss of norepinephrine producing neurons in an area of the brain called the locus ceruleus; this exists in the pons. The pons is a major structure in the upper part of your brainstem. It is involved in the control of breathing, communication between different parts of the brain, and sensations such as hearing, taste, and balance. The locus ceruleus is our main key source of norepinephrine in the brain with neurons that have widespread connections with many parts of our neural tissue. Norepinephrine is also key as it is also involved in the expression of movement which is another reason why those with PD find movement initiation difficult. Norepinephrine is also important in controlling many automatic body functions, such as the circulation of the blood. This may explain why people with PD experience dizziness when blood pressure changes on standing up (known as orthostatic hypertension).
Another characteristic of Parkinson’s Disease is the presence of what we call Lewy bodies. Lewy bodies are named after Friederich Lewy, a German neurologist who in 1912 discovered abnormal protein deposits that disrupted the brain’s normal functioning in people with PD. Lewy bodies are clumps of mis-folded proteins that occur within brain cells in PD. In the healthy brain one of these proteins is alpha-synuclein.
This plays several important roles in neurons in the brain, especially at synapses, where neurons communicate with each other. In PD, misfolded alpha-synuclein protein forms into clumps inside neurons, this occurs initially in areas of the brain that control aspects of memory and movement. This process causes neurons to work less effectively and, eventually, to die. The result is widespread damage to specific brain regions and a decline in abilities affected by those brain regions. Leading researchers believe Lewy bodies hold an important clue to the cause of Parkinson’s disease. There are several studies which have looked at silencing (or moderating) genes that have a role in the production of alpha-synuclein.
New Research on Parkinson’s Disease
Some recent research has investigated the possibility of restoring the function of dopamine producing cells in the putamen by using a naturally produced protein that is made by our glial cells called glial cell line-derived neurotrophic factor (GDNF). Overall the most common cells in the brain are glial cells and in fact, there are about ten times more glial cells than neurons. The glial cells help support, maintain the function and well-being of each neuron.
They naturally produce GDNF and this has received a lot of scientific attention. A very interesting trial was broadcasted and followed on the BCC which looked at injecting PD patients with GDNF. The actual study itself was also published in the Journal of Parkinson’s Disease in January 2019. The trail used a novel delivery system using portals to deliver and irrigate GDNF directly to the putamen. The study was encouraging. Alan Whone who helped run this study comments; ” This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s.”
Some of the latest research also adds to an unlikely growing body of evidence that PD could also possibly originate in our digestive tracts. One study recently published in the journal ‘Neuron’ was covered in the ‘Guardian’ at the end of June. These latest findings, which are based on studies in mice, back up a long-held theory that abnormally folded alpha-synuclein may originate in the gut and then spread to the brain via a nerve called the vagus nerve. The vagus nerve is a bundle of fibres that starts in the brainstem and transports signals to and from many of the body’s organs, including the gut. This study conducted a series of experiments involving more than 100 mice (oh dear, not again!).
First, they injected abnormally folded alpha-synuclein into the gut of healthy mice and tracked where the protein turned up. After one month the misfolded protein was present in several structures within the brainstem, while at three months it was also found in other regions of the brain including the amygdala and part of the midbrain rich in dopamine neurons. After seven months it was present in even more areas. The team stated that this fitted in well with the way markers of Parkinson’s disease are distributed throughout the human brain at different stages in the disease. “It supports and really provides the first experimental evidence that Parkinson’s disease can start in the gut and go up the vagus nerve,” said Ted Dawson, professor of neurology at the Johns Hopkins University school of medicine and co-author of the research.
S